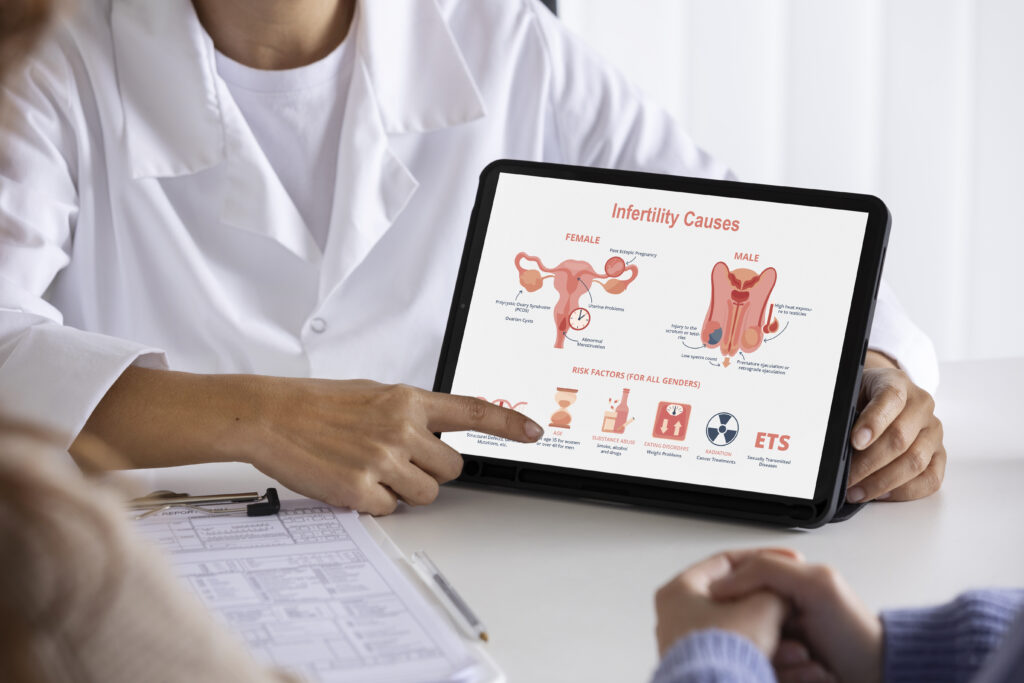
Frozen embryo transfer is a common treatment, but how we prepare the uterus for the embryo can greatly impact success. Traditionally, we’ve used vaginal progesterone, which helps prepare the uterus. However, recent research suggests that this method might not provide enough of the hormone for the entire body. Some experts believe that combining vaginal progesterone with injections could improve pregnancy rates by better supporting the body overall. Our research supports this idea and emphasizes the importance of finding the best way to use hormones during embryo transfer to increase the chances of a successful pregnancy.